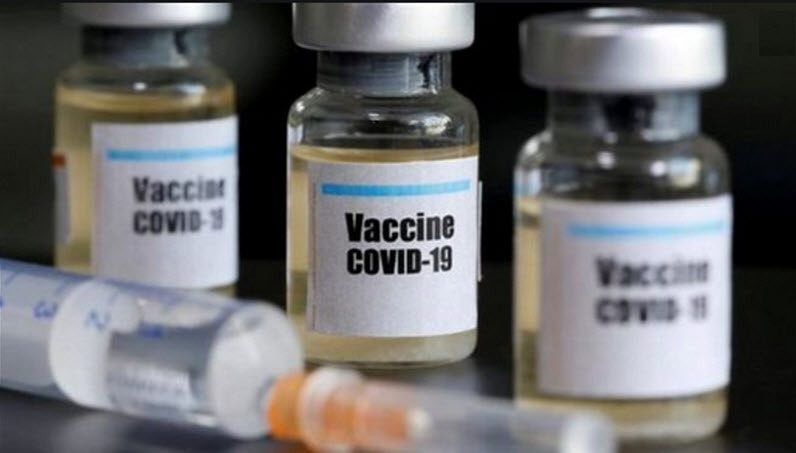
Britain is conducting trials to test a new coronavirus vaccine developed by Janssen in 17 National Institute for Health Research sites, where about 6000 volunteers participated in phase III trials of the vaccine.
According to Arabiya Net, this is the third possible Corona virus vaccine to enter clinical trials in the United Kingdom, along with vaccines from the American biotechnology company Novavax and the University of Oxford / AstraZeneca whose studies are currently underway.
The announcement of the start of clinical trials comes a week after Pfizer and Biontec revealed that early results of their vaccines showed a 90% effectiveness, according to Sky News.
Professor Saul Faust, director of clinical research at the Southampton Institute and chief investigator for the Janssen Phase III trial, told Sky News, and seen by Al Arabiya Net: The Janssen vaccine It is very similar to the Oxford / AstraZeneca vaccine in that it is a cold adapted virus that cannot multiply in the body, or sick people with colds, but it shows an increase in the protein of the immune system, allowing the secretion of antibodies to it.
Fawcett added, "All companies working to develop vaccines rely on their methodology to counteract high protein levels, so we really hope that the vaccines will work in varying proportions, and it is important that we have a number." From different vaccines from a number of different companies because we have no idea whether one vaccine will be effective for all age groups, as well as doubts about one company's capabilities in providing a vaccine that suffices the entire world.
More than 300,000 people have registered with the National Health Service's Vaccine Registry to participate in coronavirus vaccine studies.