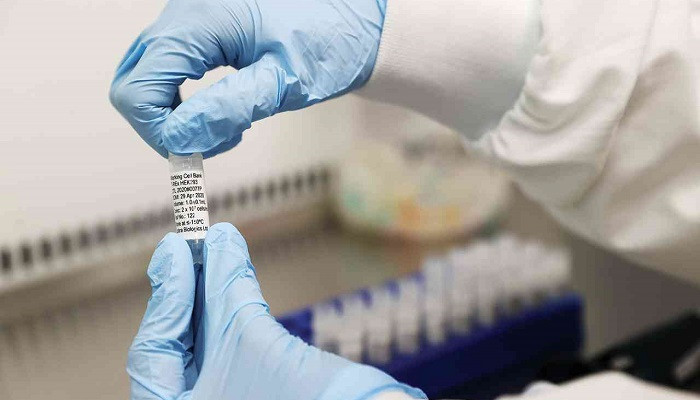
The Saudi Chemical Holding Company announced the signing of a one-year memorandum of understanding with the Russian Direct Investment Fund (RDIF); To work to provide the vaccine for Coronavirus in the Kingdom of Saudi Arabia.
and according to the company’s statement to the Saudi market (Tadawul) today, Tuesday, (Chemical) signed through its pharmaceutical sector represented by Aga Pharmaceutical Industries Ltd. (Aga Pharma) and the Saudi International Company Trading Limited (SITCO Pharma) with the Russian Direct Investment Fund RDIF (which had previously announced this year its partnership with R-Pharm and Gamaleya Research Center to produce the first Russian vaccine for Coronavirus with the possibility of exporting that to other countries) Memorandum of Understanding It is obligatory for joint cooperation regarding the Coronavirus vaccine.
The memorandum of understanding stipulated in its first stage the provision and distribution of the vaccine in the Kingdom, and then the transition to the second stage of transferring vaccine manufacturing techniques within the Kingdom, and the accompanying training and qualification National competencies. The memorandum also included the representation of the Russian Direct Investment Fund by the Saudi Chemical Holding Group before the competent government agencies in the matter of registration and clinical studies.
This memorandum is subject to obtaining the necessary approvals from the concerned authorities within the Kingdom of Saudi Arabia (the Food and Drug Authority and the relevant authorities).
This memorandum is in line with the Kingdom's Vision 2030 program, which was keen to raise support for the local content of the pharmaceutical industry through the localization of the pharmaceutical industry sector with global industrial technologies and the development of supply and distribution chains for preparations Basic pharmacokinetics. It is also compatible with the transformation program announced by the company on June 23, 2020.
The process of producing and marketing the vaccine is preceded by clinical requirements that are necessary to ensure the effectiveness and safety of the vaccine, which are still under procedure.
It is expected that if the effectiveness and safety of the vaccine is proven and all agreements related to this are terminated with the Russian side and the relevant government agencies, this will contribute positively to the company's results in the future, and it will be disclosed. About any fundamental developments in the future.
(Amazon Fun Knowledge)