<> pt = "rtl" style = "text-align: justify;"> Inovio Pharmaceuticals, a US biotechnology company, said it would start testing a possible vaccine against the Corona virus after receiving regulatory approval from clinical trials.
According to ArabiaNet, the company listed on the American money market added that it had received the approval of the Food and Drug Authority to test the new drug, thus becoming the first company in its field to receive the approval of the authorities to proceed with the development of the vaccine, according to Business Insider.
The company plans to start experimenting with 40 volunteers in Philadelphia, as well as the School of Medicine in Pennsylvania, Kansas City, and Missouri, at the Center for Drug Research.
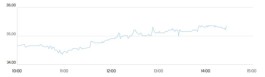
The company's shares rose about 9% in Monday's trading after the company announced the start of a possible vaccine trial, which also said it plans to increase the number of people undergoing clinical trials for about one million people by the end of this year.
The new vaccine is called INO-4800 and each participant in the clinical tests will receive two doses of the vaccine every four weeks.
The company expects to be aware of the results of the vaccine, its effect on immunity, and its ability to vaccinate against the emerging corona virus, Covid 19, by late next summer.
The company’s initial study to develop a new vaccine showed that the body had responded by creating strong immune antibodies against the deadly virus.
Last January, the company received $ 9 million in grants from a US vaccine development organization, in addition to another $ 5 million from the Bill Gates Foundation in order to accelerate production of the vaccine at a time when expectations were that the possibility of reaching an anti-virus vaccine production would range from Between a year and a year and a half.