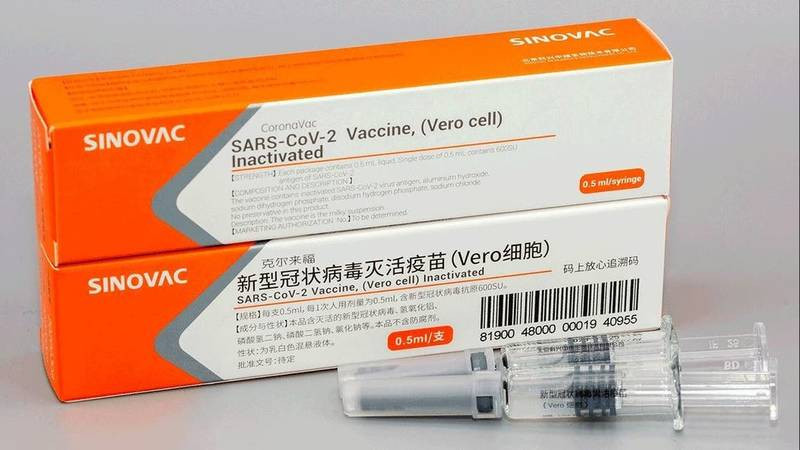
At the same time, Senovak vaccine has to adopt WHO, Egypt will begin to produce the first batch of Senovak vaccine within two weeks,
According to Arab Net, WHO agreed on the COVID-19 vaccine, which was made by the Sinovac's Chinese pharmaceutical company for emergency use. The resolution will allow Coronavac in the World Health Organization's Vaccines Participation Program, COVAX, which seeks to provide just global access to vaccinations.
Coronavac is a second Chinese vaccine receives the approval of the World Health Organization after approval of Senovarem in early May.
Dr. Khaled Mujahid, Minister of Health and Population for Information, Awareness and Speaking of the Ministry, explained that immediately after the production of the first batch of Senovak vaccine will be subject to the recent test through the analysis of the Egyptian medicine authority, pointing to Chinese experts in Egypt to oversee the process of manufacturing and transportation and technology Manufacturing, in Faxera and Egyptian Medicine Authority.
Mujahid added that Egypt is one of the first countries in the world, which has taken a move to manufacture the Virus Senovak vaccine, where Egypt has been keen to communicate and continuous coordination with the Chinese side since June last year with the participation of the Chinese embassy, even reaching the manufacturing agreement and the transfer of manufacturing technology signed between the Holding Company For biotechnology and Vacuum Vaccines and Senovak Chinese, at the Egyptian cabinet headquarters last April.
The Senovak vaccine proved an effectiveness of 91% on the public groups, according to clinical studies conducted on the vaccine in 7 different countries (Brazil - Chile - Turkey - Indonesia - China-Hong Kong - Philippines), pointing out that citizens On the Senovak vaccine will receive the second dose after 21 days (3 weeks) from receiving the first dose.