According to ArabiaNet, the company made clear in its statement of its five-point plan, as the company cooperates with the innovation system in the health care sector starting from the major drug manufacturing companies and reaching the smallest biotechnology companies, in addition to government bodies and academic institutions, with the aim of facing the health care crisis Global Caused Outbreak of Covid Disease-19.
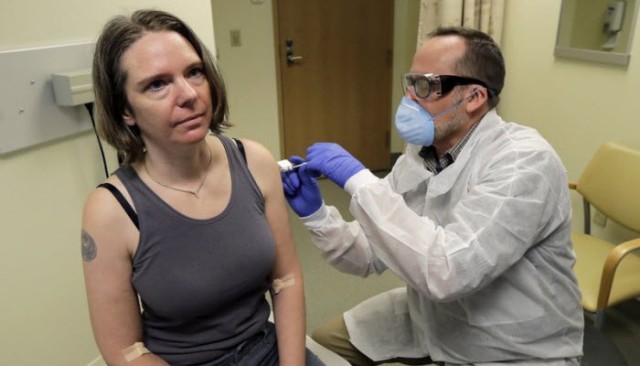
Researchers and scientists are making unremitting efforts to develop an antiviral compound to treat the emerging coronavirus, which is the primary cause of the spread of the Covid-19 pandemic, by devising a vaccine to prevent the spread of infection with the disease, as well as evaluating the effectiveness of other treatments with promising scientific capabilities to help people with the disease fight The virus.
Albert Burla, Pfizer CEO, said: Based on Pfizer's five-point plan, we face this major public health challenge by collaborating with our sector and academic institutions to develop potential new methodologies for the prevention and treatment of Covid-19 disease.
Pfizer researchers and scientists have also explored the possibilities of new uses of drugs available in the Pfizer portfolio to help people with the disease around the world. We make no effort to explore all options available to provide the community with treatment or curative treatment.
Pfizer noted that significant progress has been made in its commitment to protect humanity from this rapidly expanding pandemic, and to prepare the sector to better respond to global health crises that may arise in the future.
Pfizer confirmed the ability of one of the lead compounds and its counterparts to inhibit the protein (3CL) protein in the emerging SK virus based on the results of preliminary tests on it. Initial data also indicate that the proteinase inhibitor has been shown to be effective against the emerging corona virus.
As a result, Pfizer will conduct preclinical studies to confirm the effectiveness of the compound, including determining antiviral properties and assessing the suitability of lead particles for use in intravenous infusion therapy.
Moreover, the company is working on investing in materials that would accelerate the start of a possible clinical study of lead particles during the third quarter of 2020, i.e. three months or more before the earlier estimates for the start of the study, based on the positive completion of special pre-clinical studies Confirm the effectiveness of the compound.
Pfizer and Bayo InTech have entered into a global cooperation agreement to develop a program to produce a potential vaccine based on transmitted RNA for the prevention of Covid-19 disease. During the month of March 2020, the two companies announced the beginning of cooperation and joint action.
The two companies plan to ...