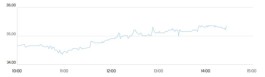
Moderna's Corona virus vaccine has received an emergency use license from the US Food and Drug Administration.
According to CNBC Arabia, the administration announced its approval of the Moderna vaccine a day after its committee approved external experts, and a week after its approval of the emergency use of the vaccine, Pfizer and its German partner Biontec.
Thousands of healthcare workers in the United States have received the Pfizer vaccine in the past seven days. It is expected to start vaccination with the Moderna vaccine within the next few days.
In a statement that two vaccines are now available to prevent Covid-19, FDA Commissioner Stephen Hahn said that the administration has taken another critical step in combating this global epidemic that causes In large numbers of hospitalized patients and deaths in the United States every day.
Moderna said it intends to apply for a full US license in 2021.
The decision represents the world's first license for the Moderna vaccine that relies on RNA technology, less than a year after the first case of coronavirus was discovered in the United States. The Pfizer vaccine is based on the same technology.
and the US Secretary of Health Alex Azar said in a statement, the authorization to use the Moderna vaccine means that we can accelerate the vaccination of frontline workers in the health sector and Americans in care facilities and ultimately eliminate this The epidemic is much faster.
The Moderna vaccine needs to be stored and shipped frozen, but not to the same degree of extreme freezing as the Pfizer-Biontech vaccine. The vaccine is given in two doses, 28 days apart.
US President Donald Trump praised the approval of Moderna's vaccine, and said on Twitter, I congratulate you. Moderna vaccine is now available.
and Moderna said it would deliver about 20 million doses to the US government this year and expected to produce between a hundred and 125 million doses in the first quarter of next year, provided that Between 85 and 100 million doses are available for the United States.
The company has entered into agreements with the US government to supply a total of 200 million doses before the end of June 2021.